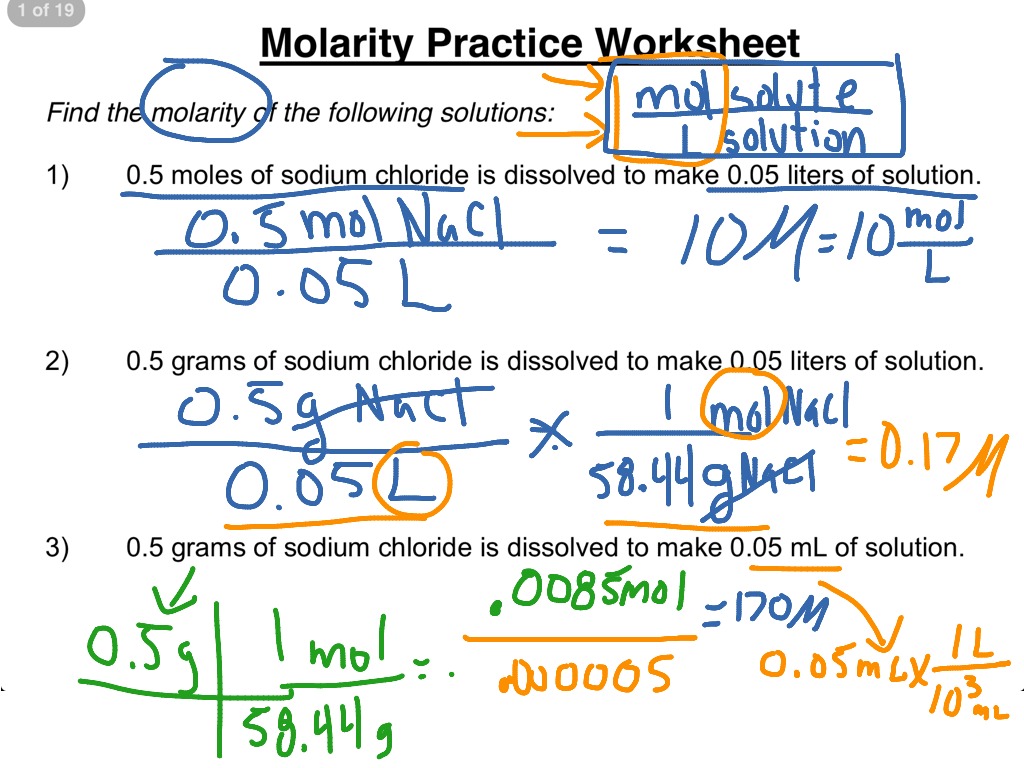
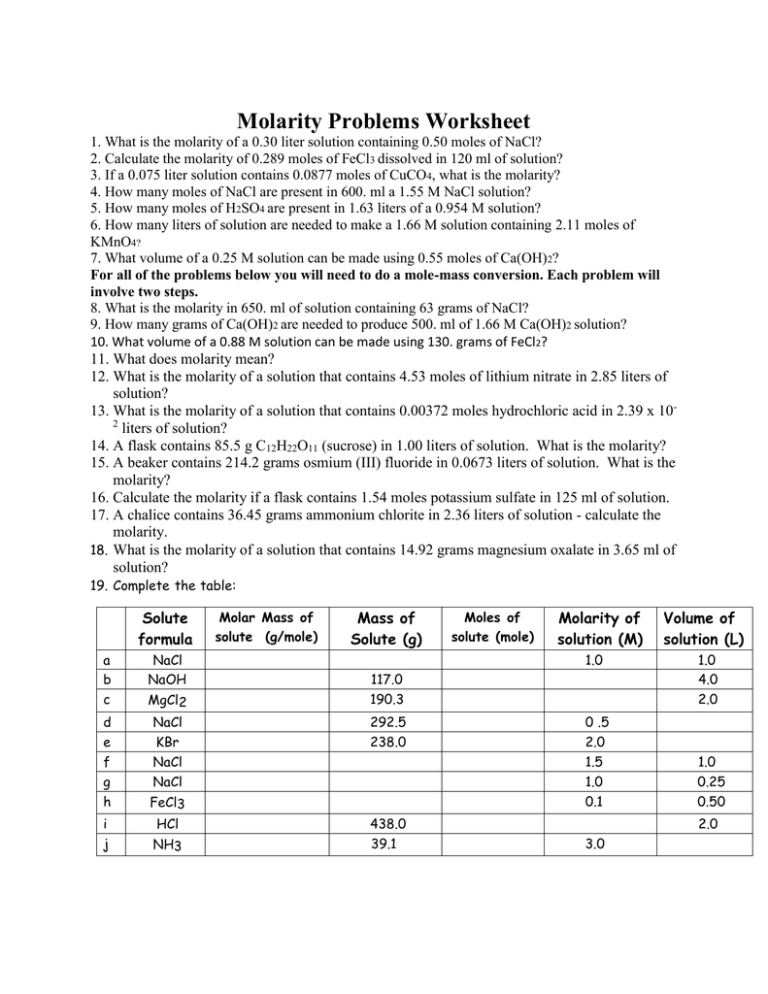
Molarity Practice Sheet - 1) 1.0 moles of potassium fluoride is dissolved to make 0.10 l of solution. What is the molarity of 2.0 l of solution made from 2.4 moles of nacl and water? Molarity of a solution is equal to the number of moles of solute divided by the number of liters of solution. What is the molarity of 500. The units, therefore are moles per liter, specifically it's. Molarity is the number of moles of solute dissolved in one liter of solution. Ml of solution made from 0.70. 1) in this problem, simply solve using. What is the molarity of the following solutions given that:
Molarity is the number of moles of solute dissolved in one liter of solution. What is the molarity of 2.0 l of solution made from 2.4 moles of nacl and water? 1) 1.0 moles of potassium fluoride is dissolved to make 0.10 l of solution. What is the molarity of the following solutions given that: 1) in this problem, simply solve using. Molarity of a solution is equal to the number of moles of solute divided by the number of liters of solution. The units, therefore are moles per liter, specifically it's. Ml of solution made from 0.70. What is the molarity of 500.
What is the molarity of 500. The units, therefore are moles per liter, specifically it's. Molarity of a solution is equal to the number of moles of solute divided by the number of liters of solution. What is the molarity of the following solutions given that: 1) in this problem, simply solve using. 1) 1.0 moles of potassium fluoride is dissolved to make 0.10 l of solution. Molarity is the number of moles of solute dissolved in one liter of solution. What is the molarity of 2.0 l of solution made from 2.4 moles of nacl and water? Ml of solution made from 0.70.
Molar Mass Practice Sheet Here are some practice problems for
Molarity is the number of moles of solute dissolved in one liter of solution. Ml of solution made from 0.70. Molarity of a solution is equal to the number of moles of solute divided by the number of liters of solution. The units, therefore are moles per liter, specifically it's. What is the molarity of 2.0 l of solution made.
Molarity Practice MS MCLARTY'S CLASSES
Molarity of a solution is equal to the number of moles of solute divided by the number of liters of solution. The units, therefore are moles per liter, specifically it's. 1) in this problem, simply solve using. What is the molarity of 500. What is the molarity of 2.0 l of solution made from 2.4 moles of nacl and water?
Molarity practice worksheet 13 Science, Chemistry, Solutions
What is the molarity of the following solutions given that: Molarity is the number of moles of solute dissolved in one liter of solution. The units, therefore are moles per liter, specifically it's. What is the molarity of 500. 1) in this problem, simply solve using.
The Ultimate Guide To Understanding The Molarity Equation In 2024
Molarity is the number of moles of solute dissolved in one liter of solution. Molarity of a solution is equal to the number of moles of solute divided by the number of liters of solution. Ml of solution made from 0.70. What is the molarity of 2.0 l of solution made from 2.4 moles of nacl and water? The units,.
Molarity Problems Worksheet Molarity Problems Worksheet Doc
1) 1.0 moles of potassium fluoride is dissolved to make 0.10 l of solution. Molarity is the number of moles of solute dissolved in one liter of solution. Molarity of a solution is equal to the number of moles of solute divided by the number of liters of solution. What is the molarity of 500. The units, therefore are moles.
Molarity Practice Problems Worksheet
Ml of solution made from 0.70. The units, therefore are moles per liter, specifically it's. Molarity is the number of moles of solute dissolved in one liter of solution. What is the molarity of 2.0 l of solution made from 2.4 moles of nacl and water? 1) 1.0 moles of potassium fluoride is dissolved to make 0.10 l of solution.
Concentration Worksheet Answer Key Solution Printable Worksheets and
What is the molarity of 2.0 l of solution made from 2.4 moles of nacl and water? Ml of solution made from 0.70. 1) 1.0 moles of potassium fluoride is dissolved to make 0.10 l of solution. The units, therefore are moles per liter, specifically it's. 1) in this problem, simply solve using.
Molarity Worksheet Chemistry Calculations & Solutions
The units, therefore are moles per liter, specifically it's. 1) in this problem, simply solve using. What is the molarity of 500. Molarity is the number of moles of solute dissolved in one liter of solution. What is the molarity of the following solutions given that:
Molarity Practice Problems Worksheets Answers
1) 1.0 moles of potassium fluoride is dissolved to make 0.10 l of solution. Ml of solution made from 0.70. What is the molarity of 500. Molarity is the number of moles of solute dissolved in one liter of solution. The units, therefore are moles per liter, specifically it's.
Molarity Molality Concentration Worksheets KEY PDF Worksheets Library
1) in this problem, simply solve using. What is the molarity of the following solutions given that: Molarity of a solution is equal to the number of moles of solute divided by the number of liters of solution. Ml of solution made from 0.70. Molarity is the number of moles of solute dissolved in one liter of solution.
Molarity Is The Number Of Moles Of Solute Dissolved In One Liter Of Solution.
What is the molarity of the following solutions given that: 1) in this problem, simply solve using. The units, therefore are moles per liter, specifically it's. Ml of solution made from 0.70.
1) 1.0 Moles Of Potassium Fluoride Is Dissolved To Make 0.10 L Of Solution.
What is the molarity of 2.0 l of solution made from 2.4 moles of nacl and water? Molarity of a solution is equal to the number of moles of solute divided by the number of liters of solution. What is the molarity of 500.