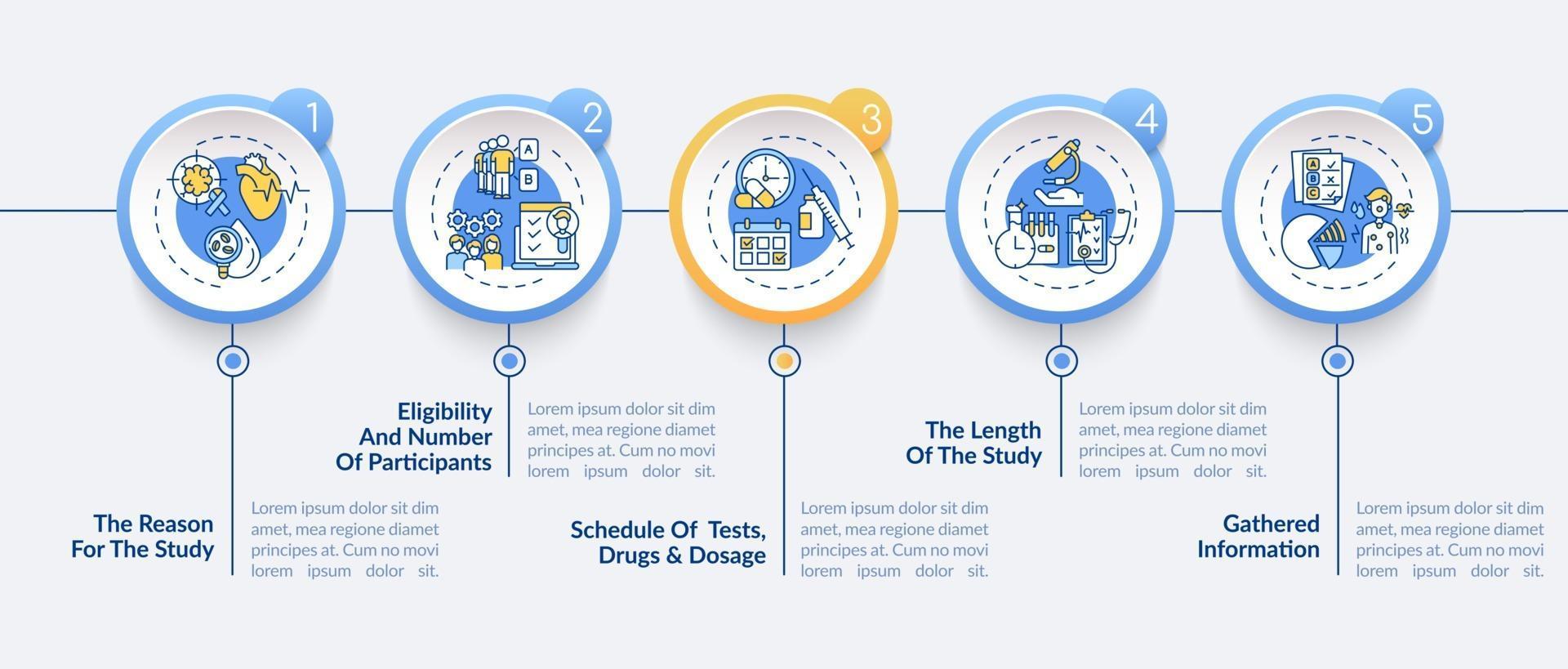
Clinical Research Protocol Template - Protocol a written account of all the procedures to be followed in a trial, which describes all the administrative, documentation, analytical and. The irb provides several protocol templates on this page. Welcome to global health trials' tools and templates library. Please note that this page has been updated for 2015 following a quality check. Nih applicants can use a template with instructional and sample text to help write clinical protocols for the following types of research:. The natural history/observational protocol template, the. Nimh encourages investigators to consider using one of the protocol templates below when developing a clinical research protocol. They follow the format of typical nih and industry multicenter protocols. Clinical trial protocol template this protocol template is designed to help research teams develop a clinical trial protocol that includes an. There are three templates to be used for observational research:
They follow the format of typical nih and industry multicenter protocols. Please note that this page has been updated for 2015 following a quality check. Protocol a written account of all the procedures to be followed in a trial, which describes all the administrative, documentation, analytical and. There are three templates to be used for observational research: Nih applicants can use a template with instructional and sample text to help write clinical protocols for the following types of research:. The irb provides several protocol templates on this page. Nimh encourages investigators to consider using one of the protocol templates below when developing a clinical research protocol. Welcome to global health trials' tools and templates library. The natural history/observational protocol template, the. Clinical trial protocol template this protocol template is designed to help research teams develop a clinical trial protocol that includes an.
Nih applicants can use a template with instructional and sample text to help write clinical protocols for the following types of research:. The natural history/observational protocol template, the. Protocol a written account of all the procedures to be followed in a trial, which describes all the administrative, documentation, analytical and. Nimh encourages investigators to consider using one of the protocol templates below when developing a clinical research protocol. They follow the format of typical nih and industry multicenter protocols. Welcome to global health trials' tools and templates library. Please note that this page has been updated for 2015 following a quality check. Clinical trial protocol template this protocol template is designed to help research teams develop a clinical trial protocol that includes an. There are three templates to be used for observational research: The irb provides several protocol templates on this page.
research protocol template
They follow the format of typical nih and industry multicenter protocols. Please note that this page has been updated for 2015 following a quality check. There are three templates to be used for observational research: Nimh encourages investigators to consider using one of the protocol templates below when developing a clinical research protocol. The natural history/observational protocol template, the.
Clinical Trial Protocol Template Word
They follow the format of typical nih and industry multicenter protocols. Welcome to global health trials' tools and templates library. There are three templates to be used for observational research: Nimh encourages investigators to consider using one of the protocol templates below when developing a clinical research protocol. Protocol a written account of all the procedures to be followed in.
Clinical trial protocol vector infographic template 2308775 Vector Art
The natural history/observational protocol template, the. Clinical trial protocol template this protocol template is designed to help research teams develop a clinical trial protocol that includes an. They follow the format of typical nih and industry multicenter protocols. Protocol a written account of all the procedures to be followed in a trial, which describes all the administrative, documentation, analytical and..
Template Device protocol
Please note that this page has been updated for 2015 following a quality check. Nimh encourages investigators to consider using one of the protocol templates below when developing a clinical research protocol. Clinical trial protocol template this protocol template is designed to help research teams develop a clinical trial protocol that includes an. The irb provides several protocol templates on.
Research Protocol Template Guide for Researchers
Welcome to global health trials' tools and templates library. Nih applicants can use a template with instructional and sample text to help write clinical protocols for the following types of research:. There are three templates to be used for observational research: Clinical trial protocol template this protocol template is designed to help research teams develop a clinical trial protocol that.
instructions for clinical research protocol template Doc Template
Welcome to global health trials' tools and templates library. Protocol a written account of all the procedures to be followed in a trial, which describes all the administrative, documentation, analytical and. The irb provides several protocol templates on this page. Nimh encourages investigators to consider using one of the protocol templates below when developing a clinical research protocol. Please note.
Phase 1 Clinical Trial Protocol Template
Nimh encourages investigators to consider using one of the protocol templates below when developing a clinical research protocol. Please note that this page has been updated for 2015 following a quality check. Clinical trial protocol template this protocol template is designed to help research teams develop a clinical trial protocol that includes an. Protocol a written account of all the.
WA Health Research Protocol Template for Clinical Trials
The irb provides several protocol templates on this page. Clinical trial protocol template this protocol template is designed to help research teams develop a clinical trial protocol that includes an. Nih applicants can use a template with instructional and sample text to help write clinical protocols for the following types of research:. Welcome to global health trials' tools and templates.
Clinical Trial Protocol Template Word
Nih applicants can use a template with instructional and sample text to help write clinical protocols for the following types of research:. The irb provides several protocol templates on this page. Clinical trial protocol template this protocol template is designed to help research teams develop a clinical trial protocol that includes an. Please note that this page has been updated.
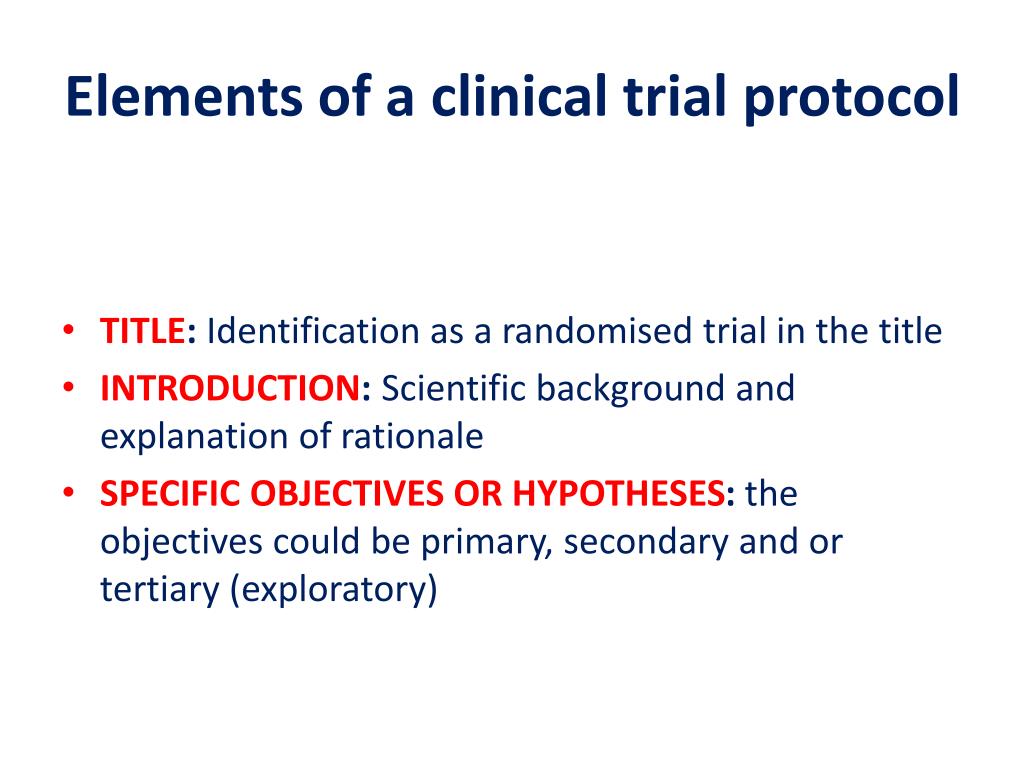
PPT Elements of a clinical trial research protocol PowerPoint
Protocol a written account of all the procedures to be followed in a trial, which describes all the administrative, documentation, analytical and. The irb provides several protocol templates on this page. Welcome to global health trials' tools and templates library. The natural history/observational protocol template, the. Please note that this page has been updated for 2015 following a quality check.
Nimh Encourages Investigators To Consider Using One Of The Protocol Templates Below When Developing A Clinical Research Protocol.
The irb provides several protocol templates on this page. Clinical trial protocol template this protocol template is designed to help research teams develop a clinical trial protocol that includes an. Welcome to global health trials' tools and templates library. Please note that this page has been updated for 2015 following a quality check.
Nih Applicants Can Use A Template With Instructional And Sample Text To Help Write Clinical Protocols For The Following Types Of Research:.
Protocol a written account of all the procedures to be followed in a trial, which describes all the administrative, documentation, analytical and. There are three templates to be used for observational research: The natural history/observational protocol template, the. They follow the format of typical nih and industry multicenter protocols.